Abstract
Background:
Induction chemotherapy for acute myeloid leukemia (AML) in adults is frequently associated with infectious complications that contribute to morbidity and mortality. Antimicrobial prophylaxis is often given during 7+3 induction to prevent such complications, but these regimens are not without risk: Clostridium difficile colitis and need for total parenteral nutrition (TPN), antimicrobial-associated toxicity, and drug resistance are considerations when choosing prophylaxis. In October 2015, Massachusetts General Hospital (MGH) implemented a change such that patients without active infection at diagnosis no longer received fluoroquinolone and fluconazole prophylaxis during induction chemotherapy. In this study, we evaluated infectious events among patients who received prophylaxis during AML induction therapy before this time point and among those who did not receive antibacterial or antifungal prophylaxis.
Methods:
We identified adults ages 18 or older with newly diagnosed AML, treated at MGH with 7 days of cytarabine and 3 days of an anthracycline ('7+3') between September 2011 and June 2017. We excluded patients with APL. Patients who were febrile at diagnosis or had a suspected infection at the start of induction received broad spectrum anti-infective coverage and were excluded from this study. Forty-five consecutive eligible patients who did not receive antibacterial or antifungal prophylaxis were evaluated as well as 90 consecutive eligible patients who received ciprofloxacin and fluconazole prophylaxis prior to October 2015. The study period spanned days 1 to 60 following the beginning of induction. Bacteremia and fungemia were defined by culture data. Colitis was defined as symptoms with radiologic findings. The incidence of each infectious event in the two groups was compared using Fisher's exact test. Time to first infectious event was compared using the log rank test. Time to neutrophil recovery in patients who achieved neutrophil recovery was compared using Wilcoxon's rank sum test.
Results:
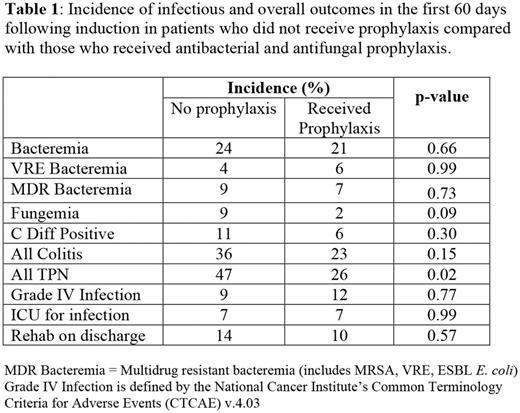
The two groups did not differ in median age at diagnosis (p = 0.88), ECOG performance score at diagnosis (p = 0.56), cytogenetic risk (p = 0.44), or rate of secondary AML (p = 0.55). There was a trend towards more fungemia in the group not receiving prophylaxis, but this did not reach statistical significance (9% vs 2%, p = 0.09). Of the 5 Candida organisms that grew in blood from patients who did not receive prophylaxis, 3 were sensitive to fluconazole and 2 sensitivities could not be obtained. There was no significant difference in the incidence of bacteremia (p = 0.66), but in those patients who did develop bacteremia, this complication occurred earlier if they did not receive prophylaxis (median 10 days vs 14 days from induction start, p = 0.03). There was no significant difference in multidrug resistant bacteremia (MRSA, VRE, or ESBL E. coli). In patients who did not receive prophylaxis and developed bacteremia, 53% of organisms isolated were gram-negative, half (50%) of which were susceptible to fluoroquinolones. In patients who received quinolone prophylaxis and developed bacteremia, 64% of organisms were gram-negative and all gram-negative organisms whose sensitivities were tested proved resistant to quinolones.
The incidence of all colitis (36% no prophylaxis vs 23%, p = 0.15) and C. diff positive stool (11% vs 6%, p = 0.30) did not differ significantly between the groups. More patients who did not receive prophylaxis required TPN (47% vs 26%, p = 0.02). Time to neutrophil count recovery > 500/mL was longer in the group receiving prophylaxis (24 vs 22 days, p = 0.02). There was no significant difference in grade IV infection, ICU admission for infection, or rehab requirement upon discharge.
Conclusions:
AML patients who did not receive a fluoroquinolone and fluconazole prophylaxis during induction had infectious complications including bacteremia earlier in their treatment course than those who received prophylaxis. The incidence of colitis or C. diff positivity did not differ between the groups, but patients not receiving prophylaxis required TPN more often. A higher percentage of positive cultures were resistant organisms among those patients given prophylaxis. Further studies are needed to continue to characterize infectious outcomes and resistance patterns based on choice of prophylaxis.
Fathi: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria; Juno: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees. Brunner: Takeda: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal